New study published in the e-Life journal - Identifies how mutations are affecting the protein's aggregation in the brain cells. The findings can be used in the future to design drugs that prevent clump of SOD1 from occurring leading to potential treatment of ALS.
JUL 13, 2021 | BY RATNESHWAR THAKUR
Amyotrophic lateral sclerosis (ALS) is an incurable brain disease that affects millions of people worldwide. Person suffering with ALS slowly loses control over voluntary movement, leading to progressive paralysis and death. Although it is not completely understood what causes ALS, a crucial protein SOD1 (Superoxide Dismutase) is known to form clumps or aggregates in brain tissue of ALS patients - seems to carry many genetic mutations.
A recent study published in e-Life by a team of researchers from India and US, led by scientists at the Indian Institute of Chemical Biology (CSIR-IICB), identifies how mutations are affecting the protein's aggregation in the brain cells. The findings can be used in the future to design drugs that prevent this clump of SOD1 from occurring leading to potential treatment of ALS.

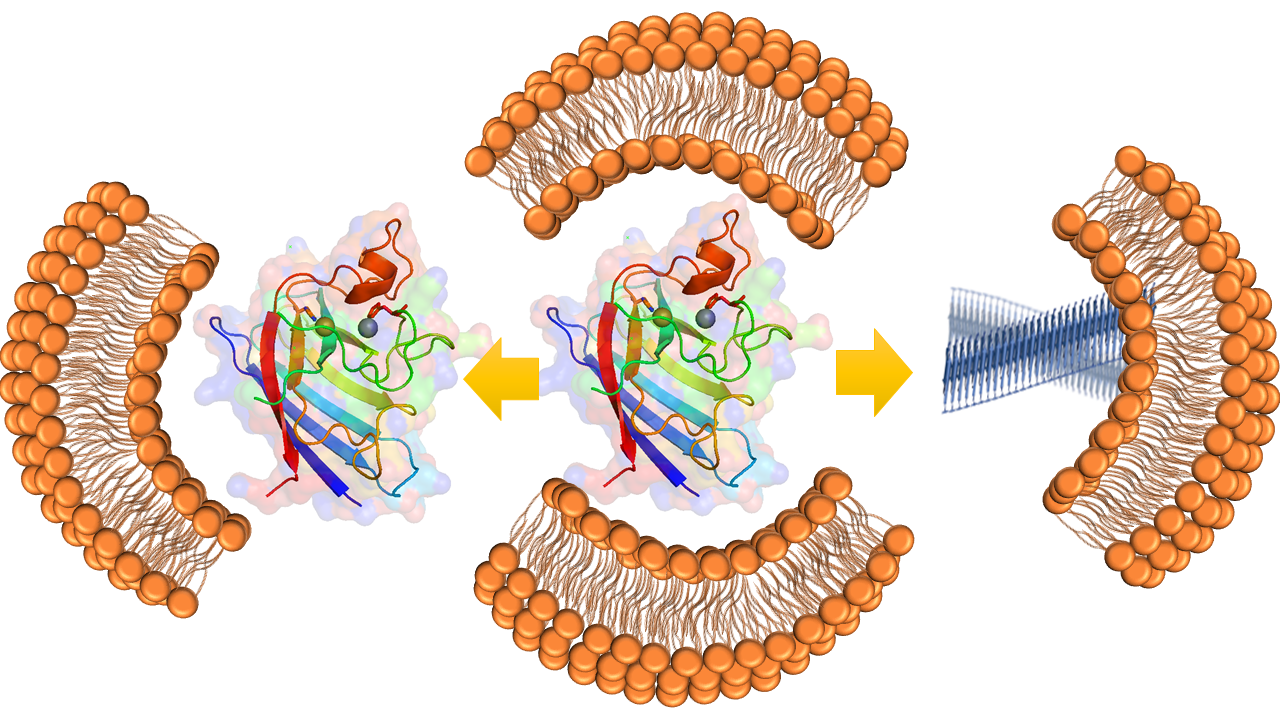
The protein SOD1 contains two metal cofactors, a Copper and a Zinc. While the cofactor copper is responsible for the physiological function of the protein - which is neutralizing harmful superoxide radicals, the other cofactor zinc provides the stability against its aggregation. The research team found that SOD1, when not bound to zinc, can attach to mitochondria membrane and forms aggregates, which are toxic.
Study suggest that the missing zinc destabilizes SOD1 resulting in its binding to mitochondrial membrane, aggregation and toxicity. “Interestingly, we find no correlation with the other cofactor Cu, which while important in the function of SOD1, shows no effect towards its aggregation,” said Achinta Sannigrahi, the lead author in this study.
“This study links a major set of mutations contributing to the different activities of a key protein, SOD1 in the brain. Out of the two ions (Copper and Zinc) known to be bound to the protein, zinc ion binding to the SOD1 seems to be critical in preventing protein clumps. This level of understanding in SOD1 protein is not available before this study. Researchers now know which region of the protein needs to be targeted for drug development against SOD1 to treat ALS,” said Rahul Roy, Chemical Engineering, Indian Institute of Science, Bangalore. He was not involved in this study.
“ALS, like other neuro-degenerative diseases, is extremely complicated. There are many hypotheses, and the aggregation of superoxide dismutase is one of the prominent one. We now know that there are hundreds of ALS mutations of this protein, while nobody knows for sure how they contribute to the severity of the disease. This paper has shown a first-time correlation that the metal cofactor zinc has a clear role in the severity associated with the mutants,” commented Atanu Biswas M.D., D.M.(Neurology), Professor, Department of Neurology, Institute of Postgraduate Medical Education & Research and Bangur Institute of Neurosciences.
“The authors validated their data using in vitro disease models; however, once it is confirmed in animals and humans, the work will guide scientists to design targeted therapeutics for the treatment of ALS,” said Timir Tripathi, Assistant Professor, Department of Biochemistry│North-Eastern Hill University, who was not associated with this study.
Research team included Achinta Sannigrahi, Sourav Chowdhury, Bidisha Das, Amrita Banerjee, Animesh Halder, Amaresh Kumar, Mohammed Saleem, Athi N Naganathan, Sanat Karmakar, and Krishnananda Chattopadhyay. The study was funded by SERB and CSIR.
Journal Reference:
The metal cofactor zinc and interacting membranes modulate SOD1 conformation-aggregation landscape in an
in vitro ALS model
Disclaimer:
SciSoup claims no competing interest.

“Our findings showed that Zn pocket surrounding regions can be targeted for design and synthesis of potential drug candidates,” he added.